Why copper? Copper as a conductor material?
Patrick Czaja | 10. May 2021
“Cables made of silver and gold-plated connectors. Is this a trend out of Hollywood?” I had to smile when this question was asked in a training course for mechatronics trainees. Haven’t you ever wondered why cables are mostly made of copper and why there are such exotics where precious metals like gold and silver are used? Copper as a conductor material has its reasons. That’s what we’re talking about today.

We all know where the term ‘conductor’ in cables comes from. Because conductors “conduct” something. Be it current or data. We have already reported in detail on the structure of conductors and cables here. Nevertheless, there is one point that keeps bothering us. Why is the strand of a cable made of copper?
Why are there cables with silver conductors and why are there gold-plated connectors, isn’t the standard actually copper?
During the training, we then dealt with this topic more intensively.
The strand of a cable
If we look at our cables very simply, in most cases they consist of several cores and a jacket that holds these cores together. The cores are the part of the cable that is also connected to a connector or end user. The core consists of a conductor, which is responsible for the flow of current, and insulation as a wrapping around the conductor. This serves to prevent the current from reaching the other cores of the cable. If the conductor consists of several wires, it is called a strand. In most cases, these are made of copper.
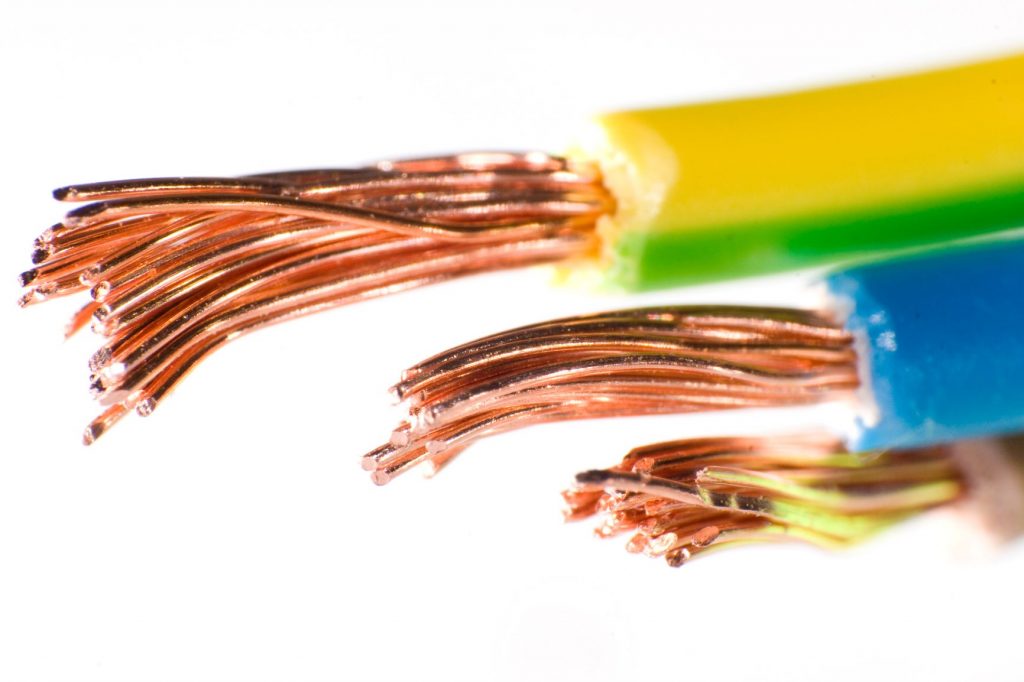
Why not aluminium as a conductor material?
To conduct electricity, we need a conductive material. These include silver, copper, gold and aluminium. The conductivity decreases according to this order. But what does conductivity actually mean? The conductivity is described, among other things, by the specific resistance of the conductor material. Figuratively, you could imagine it as a hose where the cross-section is tapered at one point. The taper represents the resistance of the conductor material. If the voltage remains the same, the current will decrease. This means that one has a lower conductivity.

Of course, we always want the best conductivity in cables, but there are other factors that play a role in the selection of the conductor material, such as durability and price.
Machines should not only operate in a fail-safe manner, but also cost-effectively. And this also includes the cables used in the applications. Although aluminium is the most cost-effective material, it is still rarely used as a conductor material.
Aluminium cannot be compared to copper in terms of stability. The wires of the strand can break more quickly, which significantly reduces the service life. However, there are also applications in which aluminium has advantages that are not only due to the lower price of the raw material. Overhead conductors are often made of aluminium. These are the cables that run across the country via the large high-voltage pylons. The advantage here is clearly the low weight.
Why not silver or gold as a conductor material?
Silver has exceptionally good conductivity, but silver and gold are simply too expensive as conductor materials. In addition, the conductivity of gold, as mentioned at the beginning, is significantly worse than that of copper. In an ordinary family home, up to one kilometre of cables are installed so that we can charge our mobile phone or keep the lights on. In an industrial plant, several kilometres of cables are quickly needed to control sensors and actuators. The budget of such plants would skyrocket if the cables were made of silver.

Why copper is used as a conductor material
Although the price of copper is always changing, there is no real alternative in terms of price-performance ratio. The copper price is a variable price. Similar to commodities such as gold or oil. Copper offers everything that a good conductor material must have. It is not only more cost-effective than the other materials, but also offers very good conductivity. The material is easy to work with due to its soft texture. Due to this flexibility, this raw material is very well suited for our cables, which are subjected to immense stress in e-chain movements. Due to these specifications, copper has established itself as the standard material for strands.
But why gold-plated connectors then?
One thing I can say right away, gold-plated connectors have nothing to do with wealth and showing off. The explanation has a purely technical background. As we have already learned above, gold has a worse conductance than copper. However, some of the electrical contacts in a connector are gold-plated. Copper, but also silver, tends to corrode when exposed to air. We know this as rusting. A layer forms which can no longer conduct the current properly. The heat generated by this can lead to fires in the connector. Covering the contacts with a thin layer of gold avoids this danger. Gold oxidises just like copper or silver, but the oxide layer is much better in electrical conductivity.
How can we help you choose the right cable?
Please contact us! We would be happy to help.
